

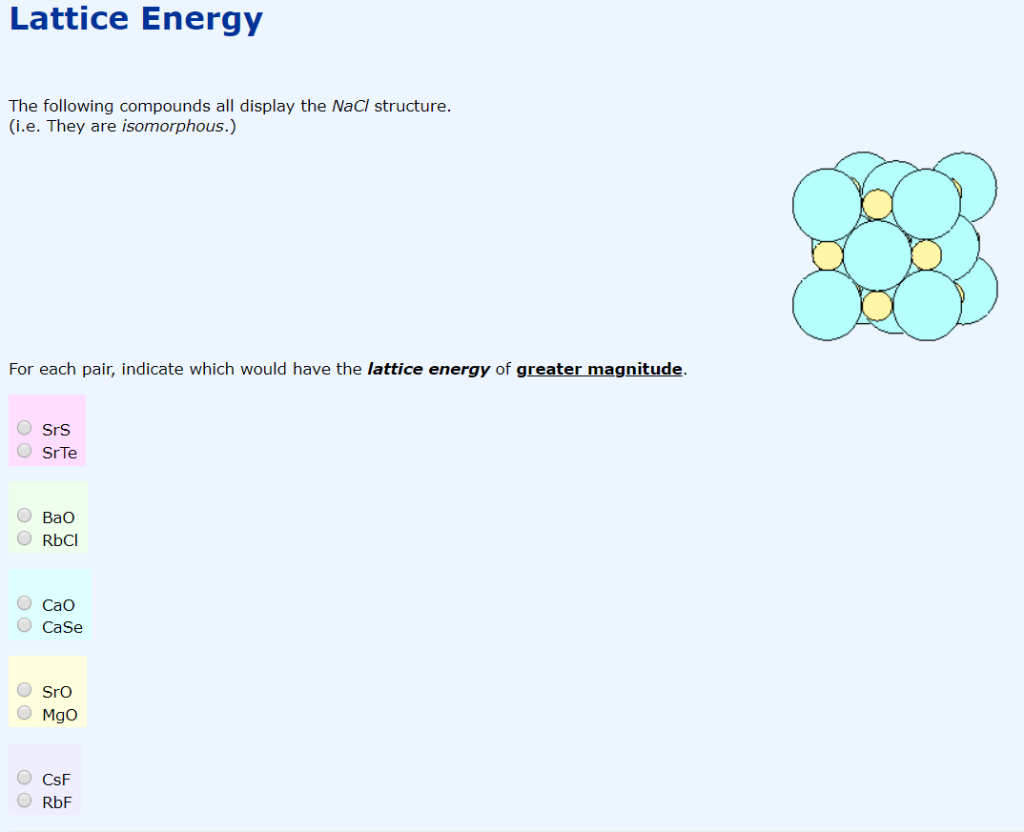
Note: Hess law’s statement, “The enthalpy change of a reaction is the same at constant volume and pressure whether it takes place in single or multiple steps long as the initial reactants and the final products remain the same.” Therefore, we defined the lattice enthalpy and calculated its value for Sodium Chloride. We begin by writing the formation reaction, which is by definition from the elemental states at 25$^)$ Now, Let’s find the lattice enthalpy of sodium chloride by using Born-Haber’s cycle. The lattice enthalpy is indirectly determined by the use of the Born-Haber Cycle. Lattice Enthalpy - The lattice enthalpy of a crystalline solid is a measure of the energy released when the ions are combined to make this compound. Now, try to answer this question accordingly. The Born-Haber cycle is a stepwise process to calculate lattice enthalpy. MgO the higher charge on Mg leads to a larger lattice energyĦ.Hint: Lattice enthalpy is related to the formation of crystalline solid from its ions.Li 2O the higher charge on O 2– leads to a larger energy additionally, Cl – is larger than O 2– this leads to a larger interionic distance in LiCl and a lower lattice energy.MgO the higher charges on Mg and O, given the similar radii of the ions, leads to a larger lattice energy.MgO selenium has larger radius than oxygen and, therefore, a larger interionic distance and thus, a larger smaller lattice energy than MgO.The compounds with the larger lattice energy are a Author for correspondence, 3rd Semester Undergraduate Student b Department of Chemistry, Ramakrishna Mission Vidyamandira. Since the lattice energy is negative in the Born-Haber cycle, this would lead to a more exothermic reaction.Ĥ. 4008 kJ/mol both ions in MgO have twice the charge of the ions in LiF the bond length is very similar and both have the same structure a quadrupling of the energy is expected based on the equation for lattice energyĥ. The smaller the radius of the anion, the shorter the interionic distance and the greater the lattice energy would be. NaCl Crystalline LatticeSodium ions (Na+) and chloride(Cl-) ions, depicted in purple and green respectively, alternate in the crystal lattice of solid NaCl.In the Born-Haber cycle, the more negative the electron affinity, the more exothermic the overall reaction. A higher electron affinity is more negative.The lower it is, the more exothermic the reaction will be. As in part (b), the bond energy is a positive energy.
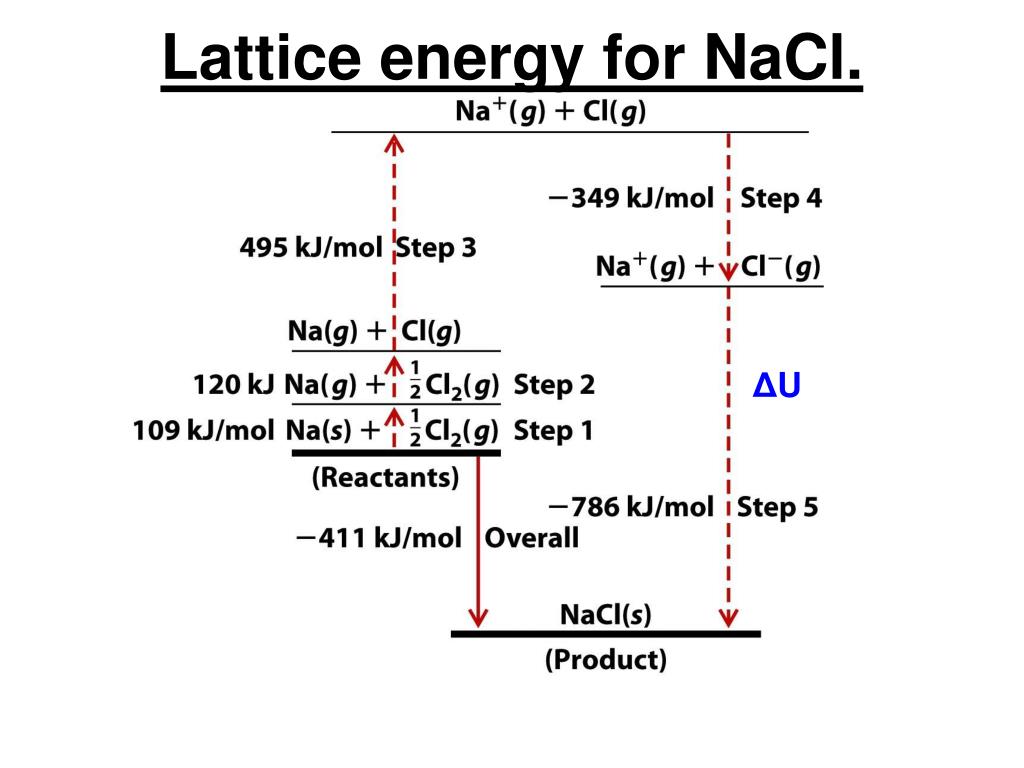
This would make the reaction more exothermic, as a smaller positive value is “more exothermic.” A lower ionization energy is a lower positive energy in the Born-Haber cycle.Since the lattice energy is negative in the Born-Haber cycle, this would lead to a more exothermic reaction. The smaller the radius of the cation, the shorter the interionic distance and the greater the lattice energy would be.Recall that the more negative the overall value, the more exothermic the reaction is. In each case, think about how it would affect the Born-Haber cycle. The answer is (d), which requires about 740 kJ/mol.ģ. U may be calculated from the Born-Haber cycle.
The lattice energy, U, is the energy required to convert the solid into separate ions. We begin with the elements in their most common states, Cs( s) and F 2( g). The Born-Haber cycle shows the relative energies of each step involved in the formation of an ionic solid from the necessary elements in their reference states.


 0 kommentar(er)
0 kommentar(er)
